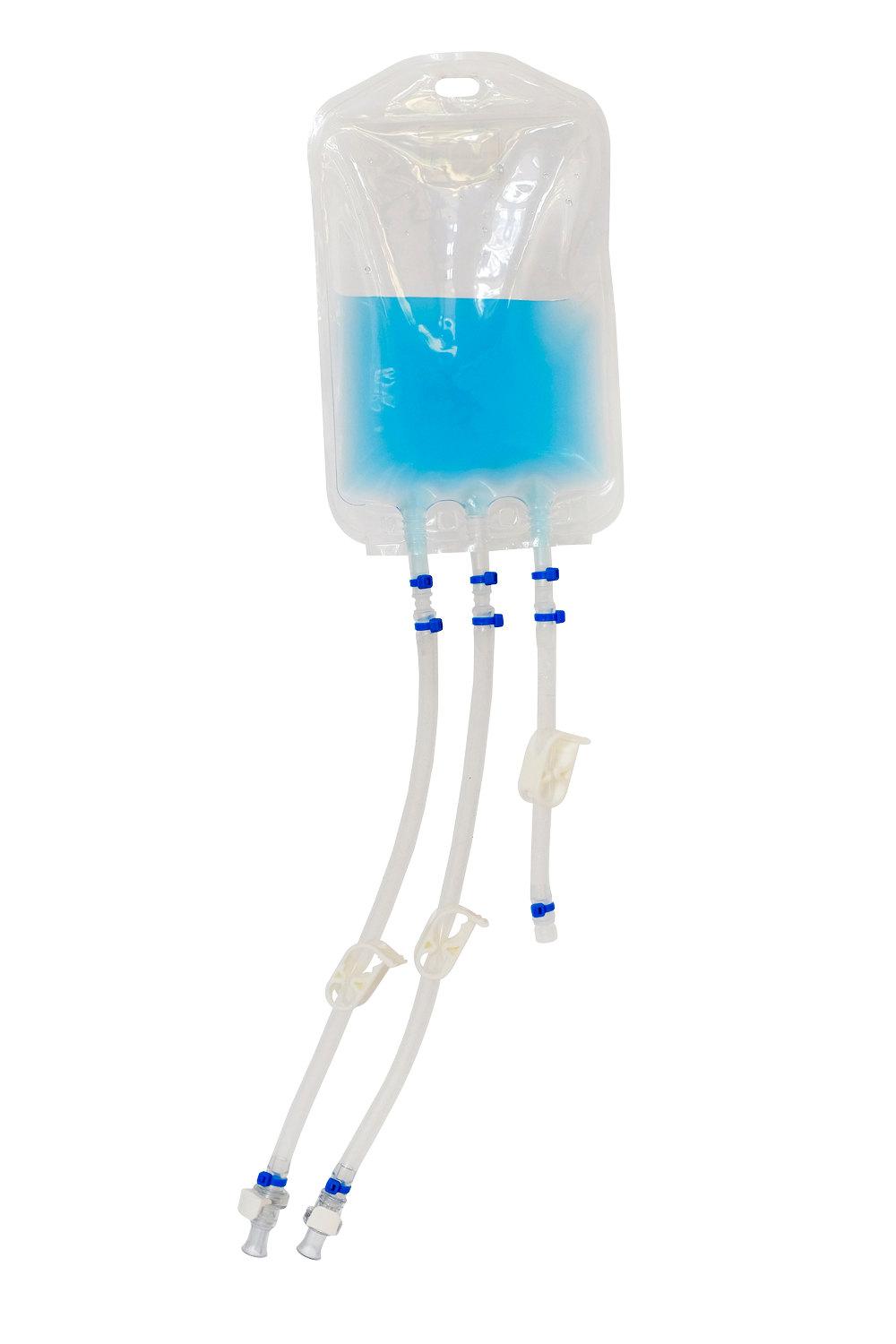
Validating a single-use system in the production of cell and gene therapy is a crucial process that ensures the quality and safety of the final product. To accomplish this, a well-defined validation strategy needs to be established, identifying critical parameters that require validation such as material compatibility, extractables and leachables, and sterilization. The following are the steps that should be taken to validate a single-use system that will be used in development and/or production of a cell and gene therapy, biologic or pharmaceutical drug product.
Define the Validation Strategy:
The first step in validating a single-use system is to define the validation strategy. This involves identifying the critical parameters that need to be validated, such as material compatibility, extractables and leachables, sterilization, package integrity, and shelf life. Material compatibility refers to the ability of the single-use components to function correctly with the other components in the system without causing any adverse reactions or degradation. Extractables and leachables refer to any substances that may be released from the single-use components and may affect the safety or efficacy of the final product. Sterilization is a critical parameter that ensures the safety of the product. Package integrity relates to seal strength and the ability of the packaging to create a sterile barrier system (SBS) and protective packaging of the single-use system. Shelf life will include accelerated aging study and an actual shelf-life test to identify how long the product can be expected to perform as designed after manufacturing and post processing. The validation strategy should also define the acceptance criteria for each parameter. These acceptance criteria should be based on relevant regulatory requirements and industry standards.
Define the Validation Study:
The next step is to design the validation study. This involves developing detailed protocol(s) that outline the procedures for testing the critical parameters identified in the validation strategy. The protocol should be developed based on regulatory guidelines, industry standards and the intended use of the product. The protocol should include the sample size, test methods, and acceptance criteria. The sample size should be statistically significant to ensure that the results are representative of the entire batch or production process. The test methods should be scientifically sound and validated. The acceptance criteria should be established based on relevant regulations and industry standards such as USP, ISO and ASTM.
Execute the Validation Study:
Once the validation study protocol has been developed, the next step is to execute the study. This involves performing the tests outlined in the protocol and documenting the results. The execution of the study should be carefully planned and performed in accordance with the protocol. All deviations from the protocol should be documented, and corrective actions should be taken to address them. The study should be performed by trained personnel in a controlled environment to ensure accuracy and reproducibility of the results.
Analyze the Data:
After the validation study has been completed, the data should be analyzed to determine whether the single-use system meets the acceptance criteria defined in the validation strategy. The data analysis should be performed by trained personnel who are experienced in statistical analysis and data interpretation. The analysis should be performed using appropriate statistical methods and software. If the acceptance criteria are not met, further testing may be required, or the single-use system may need to be modified.
Document the Validation:
The final step in validating a single-use system is to document the validation. This involves preparing a validation report that summarizes the validation strategy, study protocol, test results, and analysis of the data. The report should also include any deviations and corrective actions taken during the validation study. The validation report should be comprehensive, accurate, and written in a clear and concise manner. It should be reviewed and approved by qualified personnel and should be maintained as a part of the product history record.
In Summary
In summary, validating a single-use system in the production of cell and gene therapy requires a well-defined validation strategy, a carefully developed validation study protocol, meticulous execution of the study, data analysis, and thorough documentation. Following these steps will ensure the quality and safety of the final product.
Intellitech has broad experience in the design, development, manufacture, packaging and validation of single-use systems, partnering with independent 3rd party accredited laboratories to perform testing. We provide phase-appropriate validation studies to support customers from R&D to GMP.
Call 727-202-6419 or email sales@intellitech-inc.com to discuss your project with one of the leading companies innovating single-use systems for the life science industry.